Blog # 9 Integration of Knowledge and Application to Teaching
1.
The Experiment is Sink or Float
Materials Needed:
Tubs of water (one for every pair of children)
Small items that will float
Small items that will sink
Towels
Tubs of water (one for every pair of children)
Small items that will float
Small items that will sink
Towels
Set Up:
Fill tubs with water. Gather float and sink items, and sort them into bowls or containers.
Fill tubs with water. Gather float and sink items, and sort them into bowls or containers.
Procedure: The students will gather and record their predictions as to what will sink or float. The teacher will record in a chart for what each child thinks. She will also record the final results for each object. Give each pair of children a tub of water, and tell them they are going to test several things to see if they will float or sink. Pass each child a bottle cap, and ask them to make a prediction whether they think the cap will float or sink. Ask them to place the bottle cap in the water to see what happens. Have children test each of the items in the same manner, making a prediction first, then testing them. Do not pass out all of the items at once; instead, pass them out one at a time, randomly. Each child gets their own item to test in the water, but each pair of children shares a tub of water.
Examples of things that sink:
Pebble
Penny
Marble
Toy fish (like a math manipulative fish)
Pebble
Penny
Marble
Toy fish (like a math manipulative fish)
Examples of things that float:
Bottle Cap
Feather
Unifix cube
Small piece of paper
Feather
Unifix cube
Small piece of paper
a. This experiement/demonstration meets the following science standards:
A.4.2 When faced with a science-related problem, decide what evidence, models, or explanations previously studied can be used to better understand what is happening nowD.4.2 Group and/or classify objects and substances based on the properties of earth materials
C.4.2 Use the science content being learned to ask questions, plan investigations, make observations, make predictions, and offer explanations
C.4.6 Communicate the results of their investigations in ways their audiences will understand by using charts, graphs, drawings, written descriptions, and various other means, to display their answers
b. This experiment is designed for Preschool and Kindergarten Grade level.
c. Worksheet
Since this is a kindergarten and preschool activity the questions from the worksheet would be asked by the teacher and the children would raise their hand to share their answer.
1. What objects sank?
2. Which objects floated?
3. Why do you think some objects sank while others floated?
4. Does an object sink it is heavy or light? Circle the right answer: Heavy or Float
5. According to our chart: How many objects sank?
6. According to our chart: How many objects floated?
7. Where your guess on what would sink correct? Circle yes or no?
8. Where your guesses on what would float correct? Circle yes or no?
2. How do you feel your understanding of science, and chemistry in particular, has changed due to your experience in class?
3. What was the most Challenging concept Covered and why?
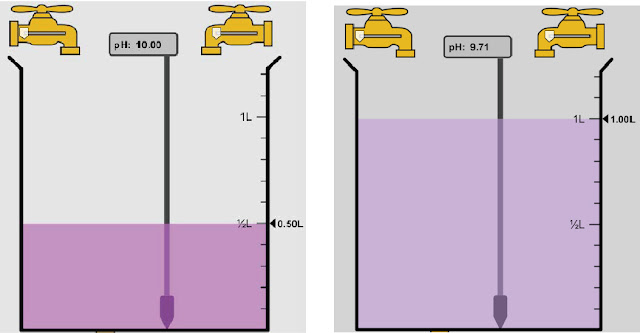
The most challenging concept we covered was acids and bases. I understood that something was either an acid or a base based on if it was above or below the Ph of seven. I had a hard time figuring out how things became this way. Like what was added or taken in to make the Ph level what it was. I did like learning about what household items or products were an acid or a base because I enjoyed learning how chemistry can relate to our world from a day to day basis.
4. How could you facilitate future learning to your students who might also find learning about science and chemistry challenging?
I could facilitate learning to my students who find this subject challenging by relating an experiment or concept to something they are already interested in to help spark interest. I would also be sure to have a very hands on experiment that the students would be active in. It may help them focus and this would allow me to walk through the area the are not understanding and answer any questions they may have with them one on one.
5. As you think about you futures in education, give three ways you think you'll be able to implement the skills you've learned in this class (It doesn't necessarily have to be content based)
- I plan to use simulations in my class like the ones that were shown in this course to either introduce a concept or further understanding of a concept for my class.